Understanding Cleanliness Standards and Testing in Ultrasonic Cleaning
Browse Volume:50 Classify:Support
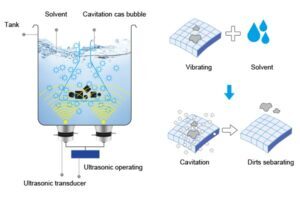
Ultrasonic cleaning is recognized for its ability to thoroughly clean objects with minimal manual effort, using high-frequency sound waves to break up contaminants on surfaces. It is widely used across industries, including healthcare, automotive, electronics, and jewelry, due to its efficiency and precision. However, to ensure that ultrasonic cleaning is truly effective, it is essential to understand how cleanliness is tested and the standards that need to be met for various applications. Cleanliness standards are crucial for maintaining the integrity of sensitive items, such as medical instruments and delicate electronic components, where contamination could have significant consequences.
The Importance of Cleanliness in Ultrasonic Cleaning
Achieving the desired level of cleanliness in ultrasonic cleaning involves mo re than just removing visible dirt or debris. In industries like healthcare and electronics, the goal is to remove microscopic contaminants such as oils, grease, bacteria, dust, and other residues that could impact the functionality, safety, or aesthetic quality of the items being cleaned. Ultrasonic cleaning must be tailored to achieve specific cleanliness criteria based on the item and the standards of the industry involved.
Cleanliness tests ensure that the ultrasonic cleaning process has removed all contaminants to meet specific standards, preventing the risks associated with inadequate cleaning. For example, a medical device that has not been cleaned correctly could cause infections, while an electronic component that retains debris could malfunction.
Types of Cleanliness Standards in Ultrasonic Cleaning
There are several standards used to assess cleanliness in ultrasonic cleaning. These standards vary depending on the industry, the type of object being cleaned, and the specific requirements for cleanliness. The following are the most commonly used cleanliness standards:
1. ISO Standards

ISO (International Organization for Standardization) provides global standards for various industries, including those for cleaning processes. Specifically, ISO 9001 and ISO 13485 are relevant in industries like manufacturing and healthcare. These standards outline requirements for quality management systems and cleanliness for medical devices, electronics, and automotive parts.
For ultrasonic cleaning, ISO 13485 is particularly significant. It sets the requirements for the cleanliness of medical devices and equipment, where contamination control is critical. In these industries, cleanliness is typically defined not just by the removal of dirt but also by microbial contamination limits.
2. FDA Guidelines for Medical Devices
In the healthcare industry, particularly with medical devices, the FDA (Food and Drug Administration) sets strict cleanliness guidelines. For devices that come into direct contact with patients, such as surgical tools, the cleaning process must adhere to FDA standards, which specify allowable levels of contamination. These guidelines focus on ensuring that sterilization, in addition to cleaning, is performed to prevent infection or cross-contamination.
For ultrasonic cleaning, the FDA ensures that the device and cleaning method effectively remove all bio-contaminants, including organic materials like blood or tissue, and minimize any risk of infection.
3. Cleanliness Levels for Electronics 
For industries dealing with electronics, such as circuit board manufacturing or the production of semiconductor devices, cleanliness is assessed in terms of ionic contamination. The presence of ionic residues can lead to circuit failures, especially when exposed to humidity or high voltages.
The cleanliness levels for electronics are often defined by the number of micrograms of ionic contamination per unit area, such as per square centimeter. For these applications, ultrasonic cleaning must be precise, removing not only visible contamination but also ionic residues that could harm the performance of delicate components.
4. Aerospace and Automotive Industry Standards
In industries like aerospace and automotive manufacturing, cleanliness standards focus on the removal of oils, greases, and particulates from metal and plastic components. These industries often use specialized ultrasonic cleaning equipment to ensure parts meet cleanliness specifications before they are used in high-precision applications, such as engines or flight systems.
The standards for these industries are typically defined by organizations such as ASTM International (formerly known as the American Society for Testing and Materials). These guidelines specify allowable limits for contamination, ensuring that the parts are free from residues that could impair performance or safety.
Testing Methods for Ultrasonic Cleaning Effectiveness
To ensure that ultrasonic cleaning achieves the necessary level of cleanliness, several testing methods are used. These tests measure the effectiveness of the cleaning process and help verify that the ultrasonic system is operating as expected. Some of the most common testing methods include:
1. Visual Inspection

Visual inspection is the simplest and most immediate method for assessing cleanliness. After an ultrasonic cleaning cycle, items can be visually inspected for any remaining visible dirt or residue. While this method is useful for initial checks, it is not sufficient for detecting microscopic contaminants that may still be present.
2. Conductivity Testing (Ionic Contamination Testing)

Ionic contamination testing is commonly used in the electronics industry to test the effectiveness of ultrasonic cleaning. This method involves measuring the conductivity of a surface to determine the amount of ionic contamination remaining after cleaning. High levels of ionic residues can lead to short-circuiting or corrosion in electronic components, so cleanliness must meet strict ionic contamination thresholds.
Conductivity testing is typically done by rinsing the cleaned object with a solution and measuring the change in conductivity. The results can indicate whether the ultrasonic cleaning process has effectively removed ionic contaminants.
3. Ultraviolet (UV) Fluorescence Testing
UV fluorescence testing is another effective method for assessing cleanliness, particularly in industries where microbial contamination is a concern. This test uses UV light to detect any residual organic matter on the surface of an item after ultrasonic cleaning. Any organic contaminants, such as proteins or fats, will fluoresce under UV light, making it easier to assess the effectiveness of the cleaning process.
4. Surface Residue Testing (Swab Tests)
Swab tests are commonly used in healthcare and food processing industries to verify that no harmful contaminants remain on a surface after cleaning. In this method, a sterile swab is used to collect any residue from the surface of an object. The swab is then analyzed in a laboratory for the presence of organic material, bacteria, or other contaminants.
Swab tests are an important part of ensuring compliance with standards such as the FDA’s requirements for medical devices and equipment. They are often used in conjunction with other cleanliness tests to ensure that the ultrasonic cleaning process has met all necessary standards.
Factors Affecting the Cleanliness of Ultrasonic Cleaning
Several factors can influence the effectiveness of ultrasonic cleaning, and understanding these factors is crucial to achieving the desired cleanliness levels. These include:
1. Frequency and Power of Ultrasonic Waves
The frequency and power of the ultrasonic waves play a critical role in the cleaning process. Lower frequencies (20-40 kHz) create larger cavitation bubbles, which are more effective at removing heavy debris from larger, tougher surfaces. However, higher frequencies (above 100 kHz) produce smaller bubbles, which are better suited for cleaning delicate or intricate items, such as jewelry or electronics.
The power of the ultrasonic waves determines how much energy is used in the cleaning process, impacting how effectively contaminants are removed. Higher power settings can speed up the cleaning process, but they may also increase the risk of damage to fragile objects. Thus, the power and frequency must be carefully chosen based on the item being cleaned and the required level of cleanliness.
2. Cleaning Solution
The type of cleaning solution used in ultrasonic cleaning can significantly impact the effectiveness of the process. While water alone may be sufficient for light cleaning, specialized solutions often contain surfactants, enzymes, or solvents that enhance the cleaning power of the ultrasonic waves. For example, in medical device cleaning, enzyme-based solutions are used to break down organic matter, such as blood or tissue residues, while non-ionic surfactants are effective in removing oils and grease.
Choosing the right solution is crucial, as some solutions may cause damage to certain materials or leave residues that can interfere with cleanliness testing. It is important to select a solution compatible with both the item being cleaned and the ultrasonic system.
3. Cleaning Time and Temperature
The duration and temperature of the cleaning cycle also affect the overall cleanliness of the item. Ultrasonic cleaning is most effective when combined with an optimal temperature, usually between 40°C and 80°C (104°F to 176°F), as this helps increase the activity of the cavitation bubbles. Longer cleaning cycles allow more time for contaminants to be removed, but excessive time may cause damage to sensitive materials.
Monitoring the time and temperature carefully is essential to achieving the desired level of cleanliness without compromising the integrity of the item.
4. Item Positioning and Load Density
Properly positioning items in the ultrasonic cleaner is vital for ensuring even cleaning. Overloading the cleaning tank or improperly positioning items can lead to uneven exposure to the ultrasonic waves, resulting in areas that are inadequately cleaned. Additionally, items should not be touching one another, as this can interfere with the cavitation process and reduce cleaning effectiveness.
Ensuring Compliance with Cleanliness Standards
To ensure that ultrasonic cleaning processes meet cleanliness standards, it is important to conduct regular testing and monitoring. Periodic audits, combined with the right testing methods, can help verify that the ultrasonic cleaning process is operating effectively and consistently. Furthermore, maintaining the ultrasonic equipment itself—such as cleaning the tank, replacing transducers, and calibrating the system—is essential for ongoing compliance with cleanliness standards.
Adhering to these guidelines helps businesses and industries maintain the highest levels of cleanliness, ensuring that items are free from contaminants and safe for use in critical
 GranboSonic
GranboSonic